Difference between revisions of "Molecular Replacement"
(→Has Phaser Solved It?) |
(→Non-default tNCS correction: Add section about tNCS arising from parallel crystallographic and non-crystallographic rotations) |
||
| (102 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
<div style="margin-left: 25px; float: right;">__TOC__</div> | <div style="margin-left: 25px; float: right;">__TOC__</div> | ||
| + | |||
| + | '''Quicklink to example scripts''' -> [[MR using keyword input]] | ||
| + | |||
| + | '''Quicklink to phaser.famos (find_alt_orig_sym_mate) documentation''' -> [[Famos]] | ||
Phaser should be able to solve most structures with the Automated Molecular Replacement mode, and this is the first mode that you should try. Give Phaser your data ([[#How to Define Data|How to Define Data]]) and your models ([[#How to Define Models|How to Define Models]]), tell Phaser what to search for, and a list of possible spacegroups (in the same point group). | Phaser should be able to solve most structures with the Automated Molecular Replacement mode, and this is the first mode that you should try. Give Phaser your data ([[#How to Define Data|How to Define Data]]) and your models ([[#How to Define Models|How to Define Models]]), tell Phaser what to search for, and a list of possible spacegroups (in the same point group). | ||
| Line 8: | Line 12: | ||
Automated Molecular Replacement combines the anisotropy correction, likelihood enhanced fast rotation function, likelihood enhanced fast translation function, packing and refinement modes for multiple search models and a set of possible spacegroups to automatically solve a structure by molecular replacement. Top solutions are output to the files FILEROOT.sol, FILEROOT.#.mtz and FILEROOT.#.pdb (where "#" refers to the sorted solution number, 1 being the best, and only 1 is output by default). Many structures can be solved by running an automated molecular replacement search with defaults, giving the ensembles that you expect to be easiest to find first. | Automated Molecular Replacement combines the anisotropy correction, likelihood enhanced fast rotation function, likelihood enhanced fast translation function, packing and refinement modes for multiple search models and a set of possible spacegroups to automatically solve a structure by molecular replacement. Top solutions are output to the files FILEROOT.sol, FILEROOT.#.mtz and FILEROOT.#.pdb (where "#" refers to the sorted solution number, 1 being the best, and only 1 is output by default). Many structures can be solved by running an automated molecular replacement search with defaults, giving the ensembles that you expect to be easiest to find first. | ||
| − | [[Image: | + | At the completion of Molecular Replacement you may wish to place your solutions on a common origin with a previous solution, for which [[Famos | Famos ]] can be used. |
| + | |||
| + | [[Image:Phaser_MR_auto2.png|Flow Diagram for Automated MR|750px]] | ||
| + | |||
| + | ==Should Phaser Solve It?== | ||
| + | The difficulty of a molecular replacement problem depends primarily on two major factors: how well the model will be able to explain the diffraction data (which depends both on the accuracy of the model and on its completeness), and how many reflections can be explained, at least in part. Each reflection provides a piece of information that helps to identify correct MR solutions. | ||
| + | |||
| + | It is possible to make a reasonable prediction of whether or not a solution will be found. If the quality of the model (its accuracy and completeness) can be estimated, then the expected contribution of each reflection to the total LLG can also be estimated. From a large battery of tests, we know that an LLG of 40 or greater usually indicates a correct solution (at least in the absence of complicating factors such as translational non-crystallographic symmetry, tNCS). Building on this understanding, if it is estimated that the LLG will be 60 or less, then Phaser will assume that the problem is a difficult one, and will implement search procedures optimised for difficult problems. | ||
| + | |||
| + | ==What Resolution of Data Should be Used?== | ||
| + | The signal for a molecular replacement solution should be very clear if the expected value of the LLG is much higher than the minimum required to be fairly certain of a solution. Currently Phaser aims for a minimum LLG of 120 and, if it is possible to achieve an even higher value, given the quality of the model and the quantity of diffraction data, then the resolution for the initial search is limited to the value required to achieve an expected LLG of 120. Data to the full resolution are still used for a final rigid-body refinement, or in a second pass if a clear solution is not found in the first attempt. | ||
| + | |||
| + | However, if the model is expected to have a large RMS error (based usually on the correlation between sequence identity and RMS error), then data to high resolution will not contribute any significant signal. Regardless of the expected LLG at the highest resolution limit, the resolution used is limited to 1.8 times the estimated RMS error of the model, because this resolution limit gives about 99% of the LLG that could be achieved. | ||
| + | |||
| + | Because Phaser implements strategies designed to solve structures with as much confidence as possible, as efficiently as possible, it is best to leave the choice of resolution to Phaser, at least in the first instance. | ||
==Has Phaser Solved It?== | ==Has Phaser Solved It?== | ||
| − | {| class="wikitable" style="text-align:center | + | {| class="wikitable" style="text-align:center" style="margin-left: 30px" |
|- | |- | ||
! TF Z-score !! Have I solved it? | ! TF Z-score !! Have I solved it? | ||
| Line 25: | Line 43: | ||
| more than 8* ||definitely | | more than 8* ||definitely | ||
|- | |- | ||
| − | + | | *''6 for 1st model in monoclinic space groups'' || | |
|} | |} | ||
| − | Ideally, | + | Ideally, a unique solution with a strong signal will be found at the end of the search. If you are searching for multiple components, then ideally the search for each component will also give a strong signal. However if the signal-to-noise of your search is low, there will be noise peaks and multiple ambiguous solutions. Signal-to-noise is judged using the '''Z-score''', which is computed by comparing the LLG values from the rotation or translation search with LLG values for a set of random rotations or translations. The mean and the RMS deviation from the mean are computed from the random set, then the Z-score for a search peak is defined as its LLG minus the mean, all divided by the RMS deviation, ''i.e. '' '''the number of standard deviations above (or below) the mean. ''' |
| − | A highly compact summary of the history of a solution is given in the | + | For a rotation function, the correct orientation may be well down the list with a Z-score (number of standard deviations above the mean value, or RFZ) under 4, and it is often not possible to identify the correct orientation until a translation function is performed and yields a clear solution. Note that the signal-to-noise of the rotation function drops with increasing number of primitive symmetry operations (the number of different orientations for symmetry-related molecules), because there is more uncertainty about how the structure factor contributions from symmetry-related copies will add up. |
| + | |||
| + | For a translation function the correct solution will generally have a Z-score (TFZ) over 5 and be well separated from the rest of the solutions. Of course, there will always be exceptions! The table gives a very rough guide to interpreting TFZ scores. This table will be updated, as we learn more from systematic molecular replacement trials. | ||
| + | |||
| + | When you are searching for multiple components, the signal may be low for the first few components but, as the model becomes more complete, the signal should become stronger. Finding a clear solution for a new component is a good sign that the partial solution to which that component was added was indeed correct. | ||
| + | |||
| + | You should always at least glance through the summary of the logfile. One thing to look for, in particular, is whether any translation solutions with a high Z-score have been rejected by the packing step. By default up to 5 percent of marker atoms (C-alpha atoms for protein) are allowed to be involved in clashes. A solution with more clashes may still be correct, and the clashes may arise only because of differences in small surface loops. If this happens, repeat the run allowing a suitable number of clashes. Note that, unless there is specific evidence in the logfile that a high TFZ-score solution is being rejected with a few clashes, it is much better to edit the model to remove the loops than to increase the number of allowed clashes. Packing criteria are a very powerful constraint on the translation function, and increasing the number of allowed clashes beyond the default will increase the search time enormously without the possibility of generating any correct solutions that would not have otherwise been found. | ||
| + | |||
| + | Note that, by default, Phaser will produce a single PDB file corresponding to the top solution found (if any), so finding a single PDB file in your output directory is not an indication that the search succeeded! You have to look, at least, at the summary of the logfile, or at the list of possible solutions in the .sol file that is produced if you run Phaser from ccp4i or command-line scripts. | ||
| + | |||
| + | ==Annotation== | ||
| + | |||
| + | A highly compact summary of the history of the statistics of a solution is given in the SOLUTION SET in the .sol file. This is a good place to start your analysis of the output. The annotation gives the Z-score of the solution at each rotation and translation function, the number of clashes in the packing, and the refined LLG. | ||
| + | |||
| + | {| class="wikitable" style="text-align:center" style="margin-left: 30px" | ||
| + | |- | ||
| + | ! Annotation !! Meaning | ||
| + | |- | ||
| + | | RFZ= || Rotation Function Z-score | ||
| + | |- | ||
| + | | TFZ= || Translation Function Z-score | ||
| + | |- | ||
| + | | PAK= || Number of packing clashes | ||
| + | |- | ||
| + | | LLG= || LLG after refinement. Will be repeated when a low resolution refinement is followed by a high resolution refinement. | ||
| + | |- | ||
| + | | TFZ== || Translation Function Z-score equivalent, only calculated for the top solution after refinement (or for the number of top files specified by TOPFILES) | ||
| + | |- | ||
| + | | RF++ || Rotation angle from previous strong solution has been used in the addition of next solution | ||
| + | |- | ||
| + | | RF*0 || Rotation angle 000 identified by low R-factor of input model | ||
| + | |- | ||
| + | | TFZ=* || First molecule in P1 (arbitrary origin, no Translation Function required) | ||
| + | |- | ||
| + | | TF*0 || Translation vector 000 identified by low R-factor of input model | ||
| + | |- | ||
| + | | (& ... & ...) || Set of TFZ PAK and LLG values for placements that were amalgamated (more than one placement from a single Translation Function) | ||
| + | |- | ||
| + | | LLG+=(... & ...) || Set of LLG values calculated during amalgamation, which will always be increasing in value | ||
| + | |- | ||
| + | | +TNCS || Components added by Translational NCS relation | ||
| + | |- | ||
| + | | *T=<i>n</i> || Solution matches template solution <i>n</i> | ||
| + | |} | ||
| + | |||
| + | Two versions of TFZ (the translation function Z-score) now appear for each component. The first ("TFZ=") is the Z-score from the actual translation search, which depends on the accuracy of the orientation used for that search. The second ("TFZ==") is the TFZ-equivalent, which indicates what the TFZ score would have been with the correct (refined) orientation. You should see the TFZ-equivalent is high at least for the final components of the solution, and that the LLG (log-likelihood gain) increases as each component of the solution is added. For example, in the case of beta-blip the annotation for the single solution output in the .sol file shows these features | ||
| + | |||
| + | SOLU SET RFZ=10.7 TFZ=24.3 PAK=0 LLG=472 TFZ==24.7 RFZ=6.4 TFZ=24.4 PAK=0 LLG=1006 TFZ==29.7 LLG=1006 TFZ==29.7 | ||
| + | SOLU 6DIM ENSE beta EULER 200.849 41.269 183.909 FRAC -0.49604 -0.15830 -0.28092 BFAC 0.00000 | ||
| + | SOLU 6DIM ENSE blip EULER 43.749 80.793 117.292 FRAC -0.12289 0.29435 -0.09266 BFAC 0.00000 | ||
| + | |||
| + | Note that the Euler angles in Phaser follow the same convention as those defined for the Crowther fast rotation function, i.e. z-y-z (rotate around the z-axis, followed by the new y-axis, followed by the new z-axis). | ||
| + | |||
| + | ==History== | ||
| + | |||
| + | A highly compact summary of the history of the peak positions of a solution is given in the SOLUTION HISTORY in the .sol file. Together with the SOLUTION SET annotation, this is useful in your analysis of the output. | ||
| + | |||
| + | {| class="wikitable" style="text-align:center" style="margin-left: 30px" | ||
| + | |- | ||
| + | ! History !! Meaning | ||
| + | |- | ||
| + | | RF/TF(r/t:n) || (r) Rotation Function peak number/(t) Translation Function peak number for the rotation function : (n) number of peak in final merged and sorted list | ||
| + | |- | ||
| + | | PAK(n:m) || (n) input solution number : (m) output solution number after packing condition applied | ||
| + | |- | ||
| + | | RNP(m,a,b,c,... : p) || All input peaks amalgamated after refinement to give output solution number (m and others): (p) output solution number | ||
| + | |- | ||
| + | | FUSE(A,B,C) || Solution numbers merged in amalgamation | ||
| + | |} | ||
| − | + | For example, in the case of beta-blip the annotation for the single solution output in the .sol file shows these features | |
| − | |||
| − | |||
| − | + | SOLU HISTORY RF/TF(1/1:1)PAK(1:1)RNP(1:1)RNP(1:1) | |
| + | SOLU 6DIM ENSE beta EULER 200.849 41.269 183.909 FRAC -0.49604 -0.15830 -0.28092 BFAC 0.00000 | ||
| + | SOLU 6DIM ENSE blip EULER 43.749 80.793 117.292 FRAC -0.12289 0.29435 -0.09266 BFAC 0.00000 | ||
| − | + | A more complicated structure solution may have | |
| − | |||
| − | |||
| − | + | SOLU HISTORY RF/TF(7/1:10)PAK(10:10)RNP(10,12,13,11,17,16,18,25,3,8,22,21,20,7,969,6,5,201,9,4,390,2,1,19:1)RNP(1:1) | |
==What to do in Difficult Cases== | ==What to do in Difficult Cases== | ||
| Line 48: | Line 132: | ||
Not every structure can be solved by molecular replacement, but the right strategy can push the limits. What to do when the default jobs fail depends on why your structure is difficult. | Not every structure can be solved by molecular replacement, but the right strategy can push the limits. What to do when the default jobs fail depends on why your structure is difficult. | ||
*'''Flexible Structure''' | *'''Flexible Structure''' | ||
| − | *:The relative orientations of the domains may be different in your crystal than in the model. If that may be the case, break the model into separate PDB files containing rigid-body units, enter these as separate ensembles, and search for them separately. If you find a convincing solution for one domain, but fail to find a solution for the next domain, you can take advantage of the knowledge that its orientation is likely to be similar to that of the first domain. The ROTAte AROUnd option of the brute rotation search can be used to restrict the search to orientations within, say, 30 degrees of that of the known domain. Allow for close approach of the domains by increasing the allowed clashes with the PACK keyword by, say, 1 for each domain break that you introduce. Alternatively, you could try generating a series of models perturbed by normal modes, with the NMAPdb keyword. One of these may duplicate the hinge motion and provide a good single model. | + | *:The relative orientations of the domains may be different in your crystal than in the model. If that may be the case, break the model into separate PDB files containing rigid-body units, enter these as separate ensembles, and search for them separately. If you find a convincing solution for one domain, but fail to find a solution for the next domain, you can take advantage of the knowledge that its orientation is likely to be similar to that of the first domain. The ROTAte AROUnd option of the brute rotation search can be used to restrict the search to orientations within, say, 30 degrees of that of the known domain. Allow for close approach of the domains by increasing the allowed clashes with the PACK keyword by, say, 1 for each domain break that you introduce. Note that it is possible to use the brute rotation search as part of the automated molecular replacement pipeline, by changing the choice of the type of rotation search. Alternatively, you could try generating a series of models perturbed by normal modes, with the NMAPdb keyword. One of these may duplicate the hinge motion and provide a good single model. |
*'''Poor or Incomplete Model''' | *'''Poor or Incomplete Model''' | ||
| − | *:Signal-to-noise is reduced by coordinate errors or incompleteness of the model. Since the rotation search has lower signal to begin with than the translation search, it is usually more severely affected. For this reason, it can be very useful to use the subsequent translation search as a way to choose among many (say 1000) orientations. | + | *:Signal-to-noise is reduced by coordinate errors or incompleteness of the model. Since the rotation search has lower signal to begin with than the translation search, it is usually more severely affected. For this reason, it can be very useful to use the subsequent translation search as a way to choose among many (say 1000) orientations. THe MR_AUTO FAST search mode automatically reduces the cutoff for accepting peaks from the fast rotation function if the decault pass does not find a solution with a high z-score, but you can manually reduce this further with the PEAKS and PURGE keywords. You can also try turning off the clustering of fast rotation function peaks because the correct orientation may sit on the shoulder of a peak in the rotation function. |
| − | *:As shown convincingly by Schwarzenbacher ''et al.'' (Schwarzenbacher, Godzik, Grzechnik & Jaroszewski, ''Acta Cryst.'' D'''60''', 1229-1236, 2004), judicious editing can make a significant difference in the quality of a distant model. In a number of tests with their data on models below 30% sequence identity, we have found that Phaser works best with a "mixed model" (non-identical sidechains longer than Ser replaced by Ser). In agreement with their results, the best models are generally derived using more sophisticated alignment protocols, such as their FFAS protocol. | + | *:As shown convincingly by Schwarzenbacher ''et al.'' (Schwarzenbacher, Godzik, Grzechnik & Jaroszewski, ''Acta Cryst.'' D'''60''', 1229-1236, 2004), judicious editing can make a significant difference in the quality of a distant model. In a number of tests with their data on models below 30% sequence identity, we have found that Phaser works best with a "mixed model" (non-identical sidechains longer than Ser replaced by Ser). In agreement with their results, the best models are generally derived using more sophisticated alignment protocols, such as their FFAS protocol. Use [http://www.phenix-online.org/documentation/sculptor.htm phenix.sculptor] to edit your model. |
*'''High Degree of Non-crystallographic Symmetry''' | *'''High Degree of Non-crystallographic Symmetry''' | ||
*:If there are clear peaks in the self-rotation function, you can expect orientations to be related by this known NCS. Methods to automatically use such information will be implemented in a future version of Phaser. In the meantime, you can work out for yourself the orientations that would be consistent with NCS and use the ROTAte AROUnd option to sample similar orientations. Alternatively, you may have an oligomeric model and expect similar NCS in the crystal. First search with the oligomeric model; if this fails, search with a monomer. If that succeeds, you can again use the ROTAte AROUnd option to force a subsequent monomer to adopt an orientation similar to the one you expect. | *:If there are clear peaks in the self-rotation function, you can expect orientations to be related by this known NCS. Methods to automatically use such information will be implemented in a future version of Phaser. In the meantime, you can work out for yourself the orientations that would be consistent with NCS and use the ROTAte AROUnd option to sample similar orientations. Alternatively, you may have an oligomeric model and expect similar NCS in the crystal. First search with the oligomeric model; if this fails, search with a monomer. If that succeeds, you can again use the ROTAte AROUnd option to force a subsequent monomer to adopt an orientation similar to the one you expect. | ||
| − | |||
| − | |||
*'''What <u>not</u> to do''' | *'''What <u>not</u> to do''' | ||
*:The automated mode of Phaser is fast when Phaser finds a high Z-score solution to your problem. When Phaser cannot find a solution with a significant Z-score, it "thrashes", meaning it maintains a list of 100-1000's of low Z-score potential solutions and tries to improve them. This can lead to exceptionally long Phaser runs (over a week of CPU time). Such runs are possible because the highly automated script allows many consecutive MR jobs to be run without you having to manually set 100-1000's of jobs running and keep track of the results. "Thrashing" generally does not produce a solution: solutions generally appear relatively quickly or not at all. It is more useful to go back and analyse your models and your data to see where improvements can be made. Your system manager will appreciate you terminating these jobs. | *:The automated mode of Phaser is fast when Phaser finds a high Z-score solution to your problem. When Phaser cannot find a solution with a significant Z-score, it "thrashes", meaning it maintains a list of 100-1000's of low Z-score potential solutions and tries to improve them. This can lead to exceptionally long Phaser runs (over a week of CPU time). Such runs are possible because the highly automated script allows many consecutive MR jobs to be run without you having to manually set 100-1000's of jobs running and keep track of the results. "Thrashing" generally does not produce a solution: solutions generally appear relatively quickly or not at all. It is more useful to go back and analyse your models and your data to see where improvements can be made. Your system manager will appreciate you terminating these jobs. | ||
| Line 70: | Line 152: | ||
Fundamental to the way in which Phaser uses MR models (either from coordinates or maps) is to estimate how the accuracy of the model falls off as a function of resolution, represented by the Sigma(A) curve. To generate the Sigma(A) curve, Phaser needs to know the RMS coordinate error expected for the model and the fraction of the scattering power in the asymmetric unit that this model contributes. | Fundamental to the way in which Phaser uses MR models (either from coordinates or maps) is to estimate how the accuracy of the model falls off as a function of resolution, represented by the Sigma(A) curve. To generate the Sigma(A) curve, Phaser needs to know the RMS coordinate error expected for the model and the fraction of the scattering power in the asymmetric unit that this model contributes. | ||
| − | + | A Babinet-style correction is used to account for the effects of disordered solvent on the completeness of the model at low resolution. | |
| − | |||
| − | |||
| − | |||
Molecular replacement models are defined with the ENSEmble keyword and the COMPosition keyword. The ENSEmble keyword gives (amongst other things) the RMS deviation for the Sigma(A) curve. The COMPosition keyword is used to deduce the fraction of the scattering power in the asymmetric unit that each ensemble contributes. The composition of the asymmetric unit is defined either by entering the molecular weights or sequences of the components in the asymmetric unit, and giving the number of copies of each. Expert users can also enter the fraction of the scattering of each component directly, although the composition must still be entered for the absolute scale calculation. Please note that the composition supplied to Phaser has to include everything in the asymmetric unit, not just what is being looked for in the current search! | Molecular replacement models are defined with the ENSEmble keyword and the COMPosition keyword. The ENSEmble keyword gives (amongst other things) the RMS deviation for the Sigma(A) curve. The COMPosition keyword is used to deduce the fraction of the scattering power in the asymmetric unit that each ensemble contributes. The composition of the asymmetric unit is defined either by entering the molecular weights or sequences of the components in the asymmetric unit, and giving the number of copies of each. Expert users can also enter the fraction of the scattering of each component directly, although the composition must still be entered for the absolute scale calculation. Please note that the composition supplied to Phaser has to include everything in the asymmetric unit, not just what is being looked for in the current search! | ||
| Line 79: | Line 158: | ||
===Building an Ensemble from Coordinates=== | ===Building an Ensemble from Coordinates=== | ||
The RMS deviation is determined directly from RMS or indirectly from IDENtity in the ENSEmble | The RMS deviation is determined directly from RMS or indirectly from IDENtity in the ENSEmble | ||
| − | keyword using the | + | keyword using a formula that depends on the sequence identity and the number of residues in the model. |
| − | + | ||
| − | RMS | + | The RMS deviation estimated from ID may be an underestimate of the true value if there is a slight conformational change between the model and target structures. To find a solution in these cases it may be necessary to increase the RMS from the default value generated from the ID, by say 0.5 Angstroms. On the other hand, when Phaser succeeds in solving a structure from a model with sequence identity much below 30%, it is often found that the fold is preserved better than the average for that level of sequence identity. So it may be worth submitting a run in which the RMS error is set at, say, 1.5, even if the sequence identity is low. The table below can be used as a guide as to the default RMS value corresponding to ID. |
| + | |||
| + | If you construct a model by homology modelling, remember that the RMS error you expect is essentially the error you expect from the template structure (if not worse!). So specify the sequence identity of the template, not of the homology model. | ||
| + | |||
| + | Only the model with the highest sequence identity is reported in the output pdb file. Also, HETATM cards in the input pdb file are ignored in the calculation of the structure factors for the ensemble, but are carried through to the output pdb file. Thus, the phases on the output mtz file (which come from the structure factors of the ensemble) do not correspond to those that would be calculated from the output pdb file, when there is more than one pdb file in an ensemble and/or the pdbfile(s) have HETATM records. | ||
| − | |||
| − | {| class="wikitable" style="text-align:center" | + | {| class="wikitable" style="text-align:center" style="margin-left: 30px" |
| + | |+ '''Initial estimate of RMS deviation in Angstrom: Number of residues in model (upper row) versus sequence identity (left column)''' | ||
|- | |- | ||
| − | ! | + | ! !! #50 !! #100 !! #200 !! #300 !! #400 !! #600 !! #850 !! #1000 !! #1500 !! #2000 |
|- | |- | ||
| − | | | + | |'''ID=0%''' || 1.579 || 1.689 || 1.875 || 2.030 || 2.164 || 2.391 || 2.625 || 2.748 || 3.093 || 3.375 |
|- | |- | ||
| − | | | + | |'''ID=10%''' || 1.356 || 1.451 || 1.610 || 1.743 || 1.858 || 2.053 || 2.255 || 2.360 || 2.657 || 2.899 |
|- | |- | ||
| − | | | + | |'''ID=20%''' || 1.165 || 1.246 || 1.383 || 1.497 || 1.596 || 1.764 || 1.936 || 2.027 || 2.281 || 2.489 |
|- | |- | ||
| − | | | + | |'''ID=30%''' || 1.000 || 1.070 || 1.188 || 1.286 || 1.371 || 1.515 || 1.663 || 1.741 || 1.959 || 2.138 |
|- | |- | ||
| − | | 40% || 1. | + | |'''ID=40%''' || 0.859 || 0.919 || 1.020 || 1.104 || 1.177 || 1.301 || 1.428 || 1.495 || 1.683 || 1.836 |
|- | |- | ||
| − | | | + | |'''ID=50%''' || 0.738 || 0.789 || 0.876 || 0.948 || 1.011 || 1.117 || 1.227 || 1.284 || 1.445 || 1.577 |
|- | |- | ||
| − | | | + | |'''ID=60%''' || 0.634 || 0.678 || 0.752 || 0.814 || 0.868 || 0.959 || 1.053 || 1.103 || 1.241 || 1.354 |
|- | |- | ||
| − | | -- | + | |'''ID=70%''' || 0.544 || 0.582 || 0.646 || 0.699 || 0.746 || 0.824 || 0.905 || 0.947 || 1.066 || 1.163 |
| − | |} | + | |- |
| + | |'''ID=80%''' || 0.467 || 0.500 || 0.555 || 0.601 || 0.640 || 0.708 || 0.777 || 0.813 || 0.915 || 0.999 | ||
| + | |- | ||
| + | |'''ID=90%''' || 0.401 || 0.429 || 0.477 || 0.516 || 0.550 || 0.608 || 0.667 || 0.698 || 0.786 || 0.858 | ||
| + | |- | ||
| + | |'''ID=100%''' || 0.345 || 0.369 || 0.409 || 0.443 || 0.472 || 0.522 || 0.573 || 0.600 || 0.675 || 0.737 | ||
| + | |} | ||
| − | |||
| − | + | ====Coordinate Editing==== | |
| + | =====HETATM/LIGANDS===== | ||
| + | Phaser ignores the scattering from HETATM records. The HETATM records are carried though to output with occupancy set to zero. Ligands will therefore not contribute to the scattering used for molecular replacement. The exceptions to this rule are the HETATM records for MSE (seleno-methionine) MSO (seleno-methionine selenoxide) CSE (seleno-cysteine) CSO (seleno-cysteine selenoxide) ALY (acetyllysine) MLY (n-dimethyl-lysine) and MLZ (n-methyl-lysine) which are used in the scattering and carried through to output with their original occupancy. If you wish to include any HETATM records in the scattering the record name use the keyword ENSE modlid HETATOM ON | ||
| − | + | =====WATER===== | |
| + | Water molecules (identified by the residue name OW WAT HOH H2O OH2 MOH WTR or TIP) are deleted from the pdb file on input, are not used in the scattering and are not carried through to file output. If you want to retain water molecules you will need to change the residue name to something other than this (e.g. WWW) so that the atoms are not identified as water. To include the water molecules in the scattering, the HETATM records will also have to be changed to ATOM records as described above. | ||
===Building an Ensemble from Electron Density=== | ===Building an Ensemble from Electron Density=== | ||
| Line 119: | Line 210: | ||
==How to Define Composition== | ==How to Define Composition== | ||
| − | The composition defines the total amount of protein and nucleic acid that you have in the asymmetric unit not the fraction of the asymmetric unit that you are | + | The composition defines the total amount of protein and nucleic acid that you have in the asymmetric unit. It is very important to include everything in the composition, not just the components that you are searching for, because Phaser needs to know what fraction of the total scattering is accounted for by each model. For the options that specify the size of a particular component (sequence, number of residues, molecular weight), you can separately define several components of the composition of the asymmetric unit and Phaser will just add them up. Note that, for these options, you can specify the composition of one copy of a component and also say how many copies of that component are expected to be present. You can also mix compositions entered by sequence, number of residues and molecular weight. When the composition is checked, Phaser will check for the plausibility of the composition you have specified, as well as multiples of that composition. |
===Default Composition=== | ===Default Composition=== | ||
For convenience, the composition defaults to 50% protein scattering by volume (the average for protein crystals). It is better to enter it explicitly, even if only to check that you have correctly deduced the probable content of your crystal. If your crystal has higher or lower solvent content than this, or contains nucleic acid, then the composition should be entered explicitly. | For convenience, the composition defaults to 50% protein scattering by volume (the average for protein crystals). It is better to enter it explicitly, even if only to check that you have correctly deduced the probable content of your crystal. If your crystal has higher or lower solvent content than this, or contains nucleic acid, then the composition should be entered explicitly. | ||
| + | ===Composition by Sequence=== | ||
| + | The composition is calculated from the amino acid sequence of the protein and the base sequence of the nucleic acid in fasta format. | ||
| + | ===Composition by Atom=== | ||
| + | Individual atoms can be added to the composition. This allows the explicit addition of heavy atoms in the structure, e.g. Fe atoms. | ||
===Composition by Solvent Content=== | ===Composition by Solvent Content=== | ||
Scattering is determined from the solvent content of the crystal, assuming that the crystal contains protein only, and the average distribution of amino acids in protein. If your crystal contains nucleic acid or your protein has an unusual amino acid distribution then the composition should be entered explicitly using the MW or sequence options. | Scattering is determined from the solvent content of the crystal, assuming that the crystal contains protein only, and the average distribution of amino acids in protein. If your crystal contains nucleic acid or your protein has an unusual amino acid distribution then the composition should be entered explicitly using the MW or sequence options. | ||
| Line 129: | Line 224: | ||
===Composition by Molecular Weight=== | ===Composition by Molecular Weight=== | ||
The composition is calculated from the molecular weight of the protein and nucleic acid assuming the protein and nucleic acid have the average distribution of amino acids and bases. If your protein or nucleic acid has an unusual amino acid or base distribution the composition should be entered by sequence. You can mix compositions entered by molecular weight with those entered by sequence. | The composition is calculated from the molecular weight of the protein and nucleic acid assuming the protein and nucleic acid have the average distribution of amino acids and bases. If your protein or nucleic acid has an unusual amino acid or base distribution the composition should be entered by sequence. You can mix compositions entered by molecular weight with those entered by sequence. | ||
| − | |||
| − | |||
===Composition by Percentage Scattering=== | ===Composition by Percentage Scattering=== | ||
The fraction scattering of each ensemble can be entered directly. The fraction scattering of each ensemble is normally automatically worked out from the average scattering from each ensemble (calculated from the pdb files if entered as coordinates, or from the protein and nucleic acid molecular weights if entered as a map) divided by the total scattering given by the composition, but entering the fraction scattering directly overrides this calculation. This option is for use when the pdb files of the models in the ensemble are unusual e.g. consist only of C-alpha atoms, or only of hydrogen atoms (as in the CLOUDS method for NMR). | The fraction scattering of each ensemble can be entered directly. The fraction scattering of each ensemble is normally automatically worked out from the average scattering from each ensemble (calculated from the pdb files if entered as coordinates, or from the protein and nucleic acid molecular weights if entered as a map) divided by the total scattering given by the composition, but entering the fraction scattering directly overrides this calculation. This option is for use when the pdb files of the models in the ensemble are unusual e.g. consist only of C-alpha atoms, or only of hydrogen atoms (as in the CLOUDS method for NMR). | ||
| + | |||
| + | ==How to Define Searches== | ||
| + | Phaser does not compare sequences you specify in the composition with the models you specify as ensembles, so you have to specify separately the number of copies of a particular sequence that you expect to be found in the asymmetric unit of your crystal and the number of copies of each ensemble you want to place in the asymmetric unit. By default, Phaser will search first for ensembles expected to yield the highest signal in the MR search (as judged by the expected LLG or eLLG calculation); if that fails to result in a clear solution, different search orders will be tested automatically. For that reason, it does not normally matter which order you use to specify the searches. There is an option to override Phaser's automatic choice of search order, but this will only rarely be useful. It is best to specify the searches for everything that you hope to find in the MR calculation in one job, as that gives Phaser the greatest scope to optimise the calculation. Note that if your crystal possesses translational non-crystallographic symmetry (tNCS), you should be searching for a number of copies of each ensemble divisible by the order of the tNCS (i.e. the number of molecules that should be related by repeated application of a translation vector). | ||
==How to Define Solutions== | ==How to Define Solutions== | ||
| Line 139: | Line 235: | ||
"PHASER.sol" files are generated by all modes (rotation function modes with VERBOSE output), and contain the current idea of potential molecular replacement solutions. | "PHASER.sol" files are generated by all modes (rotation function modes with VERBOSE output), and contain the current idea of potential molecular replacement solutions. | ||
| − | "PHASER.rlist" files are generated by the rotation function modes, and are used as input for performing translation functions. | + | "PHASER.rlist" files are generated by the rotation function modes, and are used as input for performing translation functions. |
For simple MR cases you don't really need to know how to define molecular replacement solutions. However, for difficult cases you might need to edit the files "PHASER.sol" and "PHASER.rlist" files manually | For simple MR cases you don't really need to know how to define molecular replacement solutions. However, for difficult cases you might need to edit the files "PHASER.sol" and "PHASER.rlist" files manually | ||
=== "sol" Files=== | === "sol" Files=== | ||
| − | + | SOLUtion 6DIM keywords describe Ensembles that have been oriented by a rotation search and positioned by a translation search. Each Ensemble in the asymmetric unit has its own SOLUtion keyword. When more than one (potential) molecular replacement solution is present, the solutions are separated with the SOLUTION SET keywords. | |
| − | |||
| − | When more than one (potential) molecular replacement solution is present, the solutions are separated with the SOLUTION SET keywords. | ||
==="rlist" Files=== | ==="rlist" Files=== | ||
| Line 152: | Line 246: | ||
If a partial solution is already known, then the information for the currently "known" parts of the asymmetric unit is given in the form used for the PHASER.sol file, followed by the list of trial orientations for which a translation function is to be performed. | If a partial solution is already known, then the information for the currently "known" parts of the asymmetric unit is given in the form used for the PHASER.sol file, followed by the list of trial orientations for which a translation function is to be performed. | ||
| − | |||
| − | |||
===Fixed partial structure=== | ===Fixed partial structure=== | ||
| Line 176: | Line 268: | ||
*: Enables full 6 dimensional searches, where all the solutions from the rotation function are output for testing in the translation function. This should never be necessary; it would be much faster and probably just as likely to work if the top 1000 peaks were used in this way. | *: Enables full 6 dimensional searches, where all the solutions from the rotation function are output for testing in the translation function. This should never be necessary; it would be much faster and probably just as likely to work if the top 1000 peaks were used in this way. | ||
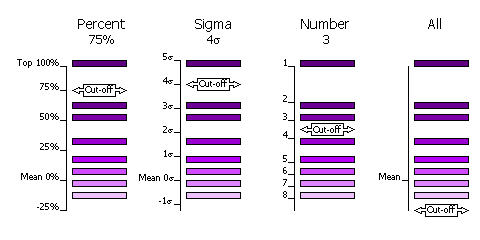
| − | [[Image:Phaser_selection.gif | + | [[Image:Phaser_selection.gif| Selection criteria]] |
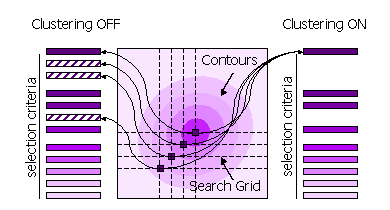
Peaks can also be clustered or not clustered prior to selection in steps 1 and 2. | Peaks can also be clustered or not clustered prior to selection in steps 1 and 2. | ||
| Line 185: | Line 277: | ||
| − | [[Image:Phaser_clustering.gif | + | [[Image:Phaser_clustering.gif| Clustering]] |
==How to Control Output== | ==How to Control Output== | ||
| Line 208: | Line 300: | ||
| HLA/HLB/HLC/HLD || Hendrickson-Lattman coefficients encoding the phase probability distribution | | HLA/HLB/HLC/HLD || Hendrickson-Lattman coefficients encoding the phase probability distribution | ||
|} | |} | ||
| + | |||
| + | ==Translational Non-crystallographic Symmetry== | ||
| + | |||
| + | <span style="color:crimson">'''*Warning*''' Solution by MR in the presence of translational non-crystallographic symmetry is not fully automated.</span> | ||
| + | |||
| + | Phaser calculates correction factors for the expected intensities in the presence of translational non-crystallographic symmetry (tNCS), and is able to solve structures with complex patterns of tNCS. '''However, the use of Phaser in the presence of tNCS requires the nature of the tNCS to be understood by the user.''' In simple cases, solution is no more difficult than solution without tNCS, but in complex cases, separate Phaser runs with tNCS turned on and off, and/or the use of different tNCS vectors, may be necessary. | ||
| + | |||
| + | The output of Phaser will help the user in detecting and understanding the tNCS, but '''the tNCS is not completely characterised by Phaser'''. The default behaviour may or may not be correct for the particular crystal under study. | ||
| + | |||
| + | Characterization of the tNCS involves understanding the number of copies of the molecule in the asymmetric unit and the translation vectors between them. Molecules related by a tNCS vector will have an associated peak in the native Patterson. Phaser calculates the native Patterson (MODE TNCS) and lists the peaks that are more than 20% of the origin peak. Any given crystal with tNCS may have one or more peaks meeting this criteria. | ||
| + | |||
| + | ===Default tNCS detection and correction=== | ||
| + | <span style="color:crimson">Documentation for Phaser-2.7.16 and above</span> | ||
| + | |||
| + | ====No tNCS==== | ||
| + | No tNCS correction is applied by default if there is | ||
| + | # no peak in the native Patterson | ||
| + | # more than one peak in the native Patterson over 20% of the origin and these peaks are not all the result of a commensurate modulation | ||
| + | |||
| + | ====Pairs of molecules==== | ||
| + | By default, if Phaser detects a peak in the native Patterson then Phaser will search for molecules in pairs related by the tNCS vector given by the peak in the native Patterson. | ||
| + | |||
| + | This will be the correct behaviour if and only if there are an even number of copies of the molecule in the asymmetric unit, clustered into two groups related by a single tNCS vector. There will only be one significant peak in the native Patterson. Fortunately, this is a reasonably common scenario. | ||
| + | |||
| + | Phaser refines the relative orientation of the molecules in the two groups (rotations of up to 10 degrees will still give rise to a significant native Patterson peak) and uses this information to generate expected intensity factors for the reflections. Solution should be straightforward, with the usual caveat for MR that there is a sufficiently good model. | ||
| + | |||
| + | Where there is a single peak in the native Patterson, it is often located at a position half way along a unit cell axis or diagonal, representing a pseudo-halving of the unit cell dimensions. However, Phaser is by no means restricted to these sorts of pseudo-cells in its handling of two-fold tNCS, and the tNCS vector can be in a general position. | ||
| + | |||
| + | ===Non-default tNCS correction=== | ||
| + | |||
| + | ====tNCS involving multimers==== | ||
| + | It is common for tNCS to arise through the combination of a crystallographic symmetry element and a nearly-parallel internal symmetry of a multimer. For example, if the local 2-fold axis of a dimer is parallel to a crystallographic 2-fold, the symmetry-related copy of monomer "B" will end up related by a pure translation to the original copy of monomer "A" from the dimer. Because Phaser handles tNCS by placing multiple copies related by the tNCS translation, the best approach in this example would be to search for two copies of the monomer. In most cases, the alternative approach of telling Phaser to ignore the tNCS will not work as well, but it may be worth testing this approach. | ||
| + | |||
| + | ====Higher order tNCS==== | ||
| + | Frequently, tNCS does not associate 2 clusters of molecules in the asymmetric unit, but rather there are 3 or more (n) clusters of molecules associated by a series of vectors that are multiples of 1, 2, 3 ... (n-1) times a basic translation vector. Where n times the basic translation vector equates to (very close to) integer multiples of unit cell axes, the tNCS represents a pseudo-cell, and this case is known as commensurate modulation. | ||
| + | |||
| + | Phaser attempts to automatically detect commensurate modulation. The peaks of the native Patterson are analyzed to find the n-fold relationship. The series will not generally have all peaks the same height. Lower peaks in the series represent relationships where the relative rotations between related molecules are larger. Missing peaks in the series may be below the default 20% of origin cut-off. This can be lowered with TNCS PATT PERCENT <x> | ||
| + | |||
| + | Phaser then sets TNCS NMOL <n> and the vector for the tNCS, and searches for ensembles in multiples of NMOL. | ||
| + | |||
| + | When there are more than two molecules related by tNCS, Phaser does not refine the orientations between the molecules related by the tNCS. | ||
| + | |||
| + | However, as for two-fold tNCS, Phaser is not restricted to these sorts of pseudo-cells and the basic tNCS vector can be in a general position, as can the number of copies. | ||
| + | |||
| + | '''The automatic detection may not give the true tNCS relationship'''. For example, the true commensurate modulation may be a factor of the NMOL automatically detected by Phaser, or there may not be commensurate modulation at all, or commensurate modulation may not be found with the default Pattesron peak height cutoff. In difficult cases, please inspect the Patterson for peaks. | ||
| + | |||
| + | ====Complex tNCS==== | ||
| + | If there are many molecules in the asymmetric unit but they are not all related by tNCS, or there are sub-groups of molecules related by different tNCS vectors, then the modulations of the expected intensities due to the tNCS will be much less significant than the cases described above. '''In these cases it is possible that structure solution will be achieved without any tNCS correction factors being applied.''' Indeed, searching for all the copies as tNCS-related multiples when some molecules are not related by tNCS will cause structure solution to fail. To turn off the automatic detection and use of tNCS use the keyword TNCS USE OFF. | ||
| + | |||
| + | If turning off the TNCS correction factors fails to give a solution, then a good approach is to proceed step-wise. Consider the highest native Patterson peak first and determine that nature of the tNCS associated with it. Use the appropriate correction factors to locate all the molecules with this tNCS. Then take the second independent native Patterson peak and apply the correction factors associated with it to find the second set of molecules, fixing the first, etc. Finally, turn TNCS off to find any orphan molecules. | ||
Latest revision as of 10:10, 20 December 2019
Contents
- 1 Automated Molecular Replacement
- 2 Should Phaser Solve It?
- 3 What Resolution of Data Should be Used?
- 4 Has Phaser Solved It?
- 5 Annotation
- 6 History
- 7 What to do in Difficult Cases
- 8 How to Define Data
- 9 How to Define Models
- 10 How to Define Composition
- 11 How to Define Searches
- 12 How to Define Solutions
- 13 How to Select Peaks
- 14 How to Control Output
- 15 Translational Non-crystallographic Symmetry
Quicklink to example scripts -> MR using keyword input
Quicklink to phaser.famos (find_alt_orig_sym_mate) documentation -> Famos
Phaser should be able to solve most structures with the Automated Molecular Replacement mode, and this is the first mode that you should try. Give Phaser your data (How to Define Data) and your models (How to Define Models), tell Phaser what to search for, and a list of possible spacegroups (in the same point group).
If this doesn't work (see Has Phaser Solved It?), you can try selecting peaks of lower significance in the rotation function in case the real orientation was not within the selection criteria. By default peaks above 75% of the top peak are selected (see How to Select Peaks). See What to do in Difficult Cases for more hints and tips. If the automated molecular replacement mode doesn't work even with non-default input you need to run the modes of Phaser separately. The possibilities are endless - you can even try exhaustive searches (translations of all orientations) if you want - but experience has shown that most structures that can be solved by Phaser can be solved by relatively simple strategies.
Automated Molecular Replacement
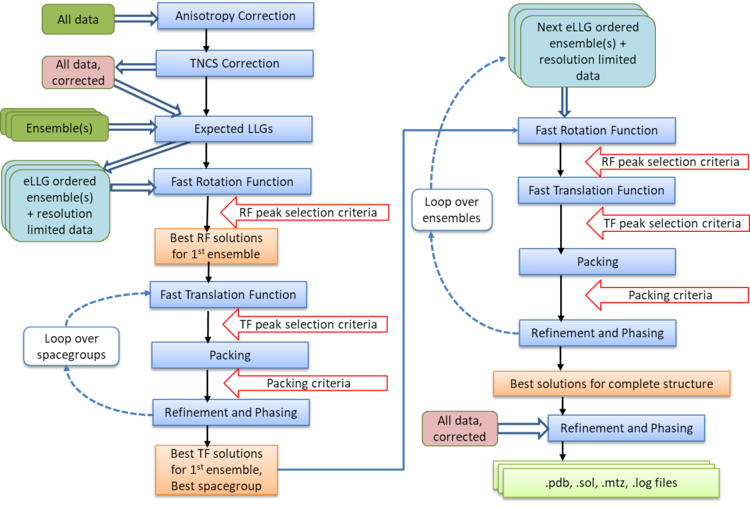
Automated Molecular Replacement combines the anisotropy correction, likelihood enhanced fast rotation function, likelihood enhanced fast translation function, packing and refinement modes for multiple search models and a set of possible spacegroups to automatically solve a structure by molecular replacement. Top solutions are output to the files FILEROOT.sol, FILEROOT.#.mtz and FILEROOT.#.pdb (where "#" refers to the sorted solution number, 1 being the best, and only 1 is output by default). Many structures can be solved by running an automated molecular replacement search with defaults, giving the ensembles that you expect to be easiest to find first.
At the completion of Molecular Replacement you may wish to place your solutions on a common origin with a previous solution, for which Famos can be used.
Should Phaser Solve It?
The difficulty of a molecular replacement problem depends primarily on two major factors: how well the model will be able to explain the diffraction data (which depends both on the accuracy of the model and on its completeness), and how many reflections can be explained, at least in part. Each reflection provides a piece of information that helps to identify correct MR solutions.
It is possible to make a reasonable prediction of whether or not a solution will be found. If the quality of the model (its accuracy and completeness) can be estimated, then the expected contribution of each reflection to the total LLG can also be estimated. From a large battery of tests, we know that an LLG of 40 or greater usually indicates a correct solution (at least in the absence of complicating factors such as translational non-crystallographic symmetry, tNCS). Building on this understanding, if it is estimated that the LLG will be 60 or less, then Phaser will assume that the problem is a difficult one, and will implement search procedures optimised for difficult problems.
What Resolution of Data Should be Used?
The signal for a molecular replacement solution should be very clear if the expected value of the LLG is much higher than the minimum required to be fairly certain of a solution. Currently Phaser aims for a minimum LLG of 120 and, if it is possible to achieve an even higher value, given the quality of the model and the quantity of diffraction data, then the resolution for the initial search is limited to the value required to achieve an expected LLG of 120. Data to the full resolution are still used for a final rigid-body refinement, or in a second pass if a clear solution is not found in the first attempt.
However, if the model is expected to have a large RMS error (based usually on the correlation between sequence identity and RMS error), then data to high resolution will not contribute any significant signal. Regardless of the expected LLG at the highest resolution limit, the resolution used is limited to 1.8 times the estimated RMS error of the model, because this resolution limit gives about 99% of the LLG that could be achieved.
Because Phaser implements strategies designed to solve structures with as much confidence as possible, as efficiently as possible, it is best to leave the choice of resolution to Phaser, at least in the first instance.
Has Phaser Solved It?
| TF Z-score | Have I solved it? |
|---|---|
| less than 5 | no |
| 5 - 6 | unlikely |
| 6 - 7 | possibly |
| 7 - 8 | probably |
| more than 8* | definitely |
| *6 for 1st model in monoclinic space groups |
Ideally, a unique solution with a strong signal will be found at the end of the search. If you are searching for multiple components, then ideally the search for each component will also give a strong signal. However if the signal-to-noise of your search is low, there will be noise peaks and multiple ambiguous solutions. Signal-to-noise is judged using the Z-score, which is computed by comparing the LLG values from the rotation or translation search with LLG values for a set of random rotations or translations. The mean and the RMS deviation from the mean are computed from the random set, then the Z-score for a search peak is defined as its LLG minus the mean, all divided by the RMS deviation, i.e. the number of standard deviations above (or below) the mean.
For a rotation function, the correct orientation may be well down the list with a Z-score (number of standard deviations above the mean value, or RFZ) under 4, and it is often not possible to identify the correct orientation until a translation function is performed and yields a clear solution. Note that the signal-to-noise of the rotation function drops with increasing number of primitive symmetry operations (the number of different orientations for symmetry-related molecules), because there is more uncertainty about how the structure factor contributions from symmetry-related copies will add up.
For a translation function the correct solution will generally have a Z-score (TFZ) over 5 and be well separated from the rest of the solutions. Of course, there will always be exceptions! The table gives a very rough guide to interpreting TFZ scores. This table will be updated, as we learn more from systematic molecular replacement trials.
When you are searching for multiple components, the signal may be low for the first few components but, as the model becomes more complete, the signal should become stronger. Finding a clear solution for a new component is a good sign that the partial solution to which that component was added was indeed correct.
You should always at least glance through the summary of the logfile. One thing to look for, in particular, is whether any translation solutions with a high Z-score have been rejected by the packing step. By default up to 5 percent of marker atoms (C-alpha atoms for protein) are allowed to be involved in clashes. A solution with more clashes may still be correct, and the clashes may arise only because of differences in small surface loops. If this happens, repeat the run allowing a suitable number of clashes. Note that, unless there is specific evidence in the logfile that a high TFZ-score solution is being rejected with a few clashes, it is much better to edit the model to remove the loops than to increase the number of allowed clashes. Packing criteria are a very powerful constraint on the translation function, and increasing the number of allowed clashes beyond the default will increase the search time enormously without the possibility of generating any correct solutions that would not have otherwise been found.
Note that, by default, Phaser will produce a single PDB file corresponding to the top solution found (if any), so finding a single PDB file in your output directory is not an indication that the search succeeded! You have to look, at least, at the summary of the logfile, or at the list of possible solutions in the .sol file that is produced if you run Phaser from ccp4i or command-line scripts.
Annotation
A highly compact summary of the history of the statistics of a solution is given in the SOLUTION SET in the .sol file. This is a good place to start your analysis of the output. The annotation gives the Z-score of the solution at each rotation and translation function, the number of clashes in the packing, and the refined LLG.
| Annotation | Meaning |
|---|---|
| RFZ= | Rotation Function Z-score |
| TFZ= | Translation Function Z-score |
| PAK= | Number of packing clashes |
| LLG= | LLG after refinement. Will be repeated when a low resolution refinement is followed by a high resolution refinement. |
| TFZ== | Translation Function Z-score equivalent, only calculated for the top solution after refinement (or for the number of top files specified by TOPFILES) |
| RF++ | Rotation angle from previous strong solution has been used in the addition of next solution |
| RF*0 | Rotation angle 000 identified by low R-factor of input model |
| TFZ=* | First molecule in P1 (arbitrary origin, no Translation Function required) |
| TF*0 | Translation vector 000 identified by low R-factor of input model |
| (& ... & ...) | Set of TFZ PAK and LLG values for placements that were amalgamated (more than one placement from a single Translation Function) |
| LLG+=(... & ...) | Set of LLG values calculated during amalgamation, which will always be increasing in value |
| +TNCS | Components added by Translational NCS relation |
| *T=n | Solution matches template solution n |
Two versions of TFZ (the translation function Z-score) now appear for each component. The first ("TFZ=") is the Z-score from the actual translation search, which depends on the accuracy of the orientation used for that search. The second ("TFZ==") is the TFZ-equivalent, which indicates what the TFZ score would have been with the correct (refined) orientation. You should see the TFZ-equivalent is high at least for the final components of the solution, and that the LLG (log-likelihood gain) increases as each component of the solution is added. For example, in the case of beta-blip the annotation for the single solution output in the .sol file shows these features
SOLU SET RFZ=10.7 TFZ=24.3 PAK=0 LLG=472 TFZ==24.7 RFZ=6.4 TFZ=24.4 PAK=0 LLG=1006 TFZ==29.7 LLG=1006 TFZ==29.7 SOLU 6DIM ENSE beta EULER 200.849 41.269 183.909 FRAC -0.49604 -0.15830 -0.28092 BFAC 0.00000 SOLU 6DIM ENSE blip EULER 43.749 80.793 117.292 FRAC -0.12289 0.29435 -0.09266 BFAC 0.00000
Note that the Euler angles in Phaser follow the same convention as those defined for the Crowther fast rotation function, i.e. z-y-z (rotate around the z-axis, followed by the new y-axis, followed by the new z-axis).
History
A highly compact summary of the history of the peak positions of a solution is given in the SOLUTION HISTORY in the .sol file. Together with the SOLUTION SET annotation, this is useful in your analysis of the output.
| History | Meaning |
|---|---|
| RF/TF(r/t:n) | (r) Rotation Function peak number/(t) Translation Function peak number for the rotation function : (n) number of peak in final merged and sorted list |
| PAK(n:m) | (n) input solution number : (m) output solution number after packing condition applied |
| RNP(m,a,b,c,... : p) | All input peaks amalgamated after refinement to give output solution number (m and others): (p) output solution number |
| FUSE(A,B,C) | Solution numbers merged in amalgamation |
For example, in the case of beta-blip the annotation for the single solution output in the .sol file shows these features
SOLU HISTORY RF/TF(1/1:1)PAK(1:1)RNP(1:1)RNP(1:1) SOLU 6DIM ENSE beta EULER 200.849 41.269 183.909 FRAC -0.49604 -0.15830 -0.28092 BFAC 0.00000 SOLU 6DIM ENSE blip EULER 43.749 80.793 117.292 FRAC -0.12289 0.29435 -0.09266 BFAC 0.00000
A more complicated structure solution may have
SOLU HISTORY RF/TF(7/1:10)PAK(10:10)RNP(10,12,13,11,17,16,18,25,3,8,22,21,20,7,969,6,5,201,9,4,390,2,1,19:1)RNP(1:1)
What to do in Difficult Cases
Not every structure can be solved by molecular replacement, but the right strategy can push the limits. What to do when the default jobs fail depends on why your structure is difficult.
- Flexible Structure
- The relative orientations of the domains may be different in your crystal than in the model. If that may be the case, break the model into separate PDB files containing rigid-body units, enter these as separate ensembles, and search for them separately. If you find a convincing solution for one domain, but fail to find a solution for the next domain, you can take advantage of the knowledge that its orientation is likely to be similar to that of the first domain. The ROTAte AROUnd option of the brute rotation search can be used to restrict the search to orientations within, say, 30 degrees of that of the known domain. Allow for close approach of the domains by increasing the allowed clashes with the PACK keyword by, say, 1 for each domain break that you introduce. Note that it is possible to use the brute rotation search as part of the automated molecular replacement pipeline, by changing the choice of the type of rotation search. Alternatively, you could try generating a series of models perturbed by normal modes, with the NMAPdb keyword. One of these may duplicate the hinge motion and provide a good single model.
- Poor or Incomplete Model
- Signal-to-noise is reduced by coordinate errors or incompleteness of the model. Since the rotation search has lower signal to begin with than the translation search, it is usually more severely affected. For this reason, it can be very useful to use the subsequent translation search as a way to choose among many (say 1000) orientations. THe MR_AUTO FAST search mode automatically reduces the cutoff for accepting peaks from the fast rotation function if the decault pass does not find a solution with a high z-score, but you can manually reduce this further with the PEAKS and PURGE keywords. You can also try turning off the clustering of fast rotation function peaks because the correct orientation may sit on the shoulder of a peak in the rotation function.
- As shown convincingly by Schwarzenbacher et al. (Schwarzenbacher, Godzik, Grzechnik & Jaroszewski, Acta Cryst. D60, 1229-1236, 2004), judicious editing can make a significant difference in the quality of a distant model. In a number of tests with their data on models below 30% sequence identity, we have found that Phaser works best with a "mixed model" (non-identical sidechains longer than Ser replaced by Ser). In agreement with their results, the best models are generally derived using more sophisticated alignment protocols, such as their FFAS protocol. Use phenix.sculptor to edit your model.
- High Degree of Non-crystallographic Symmetry
- If there are clear peaks in the self-rotation function, you can expect orientations to be related by this known NCS. Methods to automatically use such information will be implemented in a future version of Phaser. In the meantime, you can work out for yourself the orientations that would be consistent with NCS and use the ROTAte AROUnd option to sample similar orientations. Alternatively, you may have an oligomeric model and expect similar NCS in the crystal. First search with the oligomeric model; if this fails, search with a monomer. If that succeeds, you can again use the ROTAte AROUnd option to force a subsequent monomer to adopt an orientation similar to the one you expect.
- What not to do
- The automated mode of Phaser is fast when Phaser finds a high Z-score solution to your problem. When Phaser cannot find a solution with a significant Z-score, it "thrashes", meaning it maintains a list of 100-1000's of low Z-score potential solutions and tries to improve them. This can lead to exceptionally long Phaser runs (over a week of CPU time). Such runs are possible because the highly automated script allows many consecutive MR jobs to be run without you having to manually set 100-1000's of jobs running and keep track of the results. "Thrashing" generally does not produce a solution: solutions generally appear relatively quickly or not at all. It is more useful to go back and analyse your models and your data to see where improvements can be made. Your system manager will appreciate you terminating these jobs.
- It is also not a good idea to effectively remove the packing test. Unless there is specific evidence in the logfile that a high TF-function Z-score solution is being rejected with a few clashes, it is much better to edit the model to remove the loops than to increase the number of allowed clashes. Packing criteria are a very powerful constraint on the translation function, and increasing the number of allowed clashes beyond a few (e.g. 1-5) will increase the search time enormously without the possibility of generating any correct solutions that would not have otherwise been found.
- Other suggestions
- Phaser has powerful input, output and scripting facilities that allow a large number of possibilities for altering default behaviour and forcing Phaser to do what you think it should. However, you will need to read the information in the manual below to take advantage of these facilities!
How to Define Data
You need to tell Phaser the name of the mtz file containing your data and the columns in the mtz file to be used using the HKLIn and LABIn keywords. Additional keywords (BINS CELL OUTLier RESOlution SPACegroup) define how the data are used.
How to Define Models
Phaser must be given the models that it will use for molecular replacement. A model in Phaser is referred to as an "ensemble", even when it is described by a single file. This is because it is possible to provide a set of aligned structures as an ensemble, from which a statistically-weighted averaged model is calculated. A molecular replacement model is provided either as one or more aligned pdb files, or as an electron density map, entered as structure factors in an mtz file. Each ensemble is treated as a separate type of rigid body to be placed in the molecular replacement solution. An ensemble should only be defined once, even if there are several copies of the molecule in the asymmetric unit.
Fundamental to the way in which Phaser uses MR models (either from coordinates or maps) is to estimate how the accuracy of the model falls off as a function of resolution, represented by the Sigma(A) curve. To generate the Sigma(A) curve, Phaser needs to know the RMS coordinate error expected for the model and the fraction of the scattering power in the asymmetric unit that this model contributes.
A Babinet-style correction is used to account for the effects of disordered solvent on the completeness of the model at low resolution.
Molecular replacement models are defined with the ENSEmble keyword and the COMPosition keyword. The ENSEmble keyword gives (amongst other things) the RMS deviation for the Sigma(A) curve. The COMPosition keyword is used to deduce the fraction of the scattering power in the asymmetric unit that each ensemble contributes. The composition of the asymmetric unit is defined either by entering the molecular weights or sequences of the components in the asymmetric unit, and giving the number of copies of each. Expert users can also enter the fraction of the scattering of each component directly, although the composition must still be entered for the absolute scale calculation. Please note that the composition supplied to Phaser has to include everything in the asymmetric unit, not just what is being looked for in the current search!
Building an Ensemble from Coordinates
The RMS deviation is determined directly from RMS or indirectly from IDENtity in the ENSEmble keyword using a formula that depends on the sequence identity and the number of residues in the model.
The RMS deviation estimated from ID may be an underestimate of the true value if there is a slight conformational change between the model and target structures. To find a solution in these cases it may be necessary to increase the RMS from the default value generated from the ID, by say 0.5 Angstroms. On the other hand, when Phaser succeeds in solving a structure from a model with sequence identity much below 30%, it is often found that the fold is preserved better than the average for that level of sequence identity. So it may be worth submitting a run in which the RMS error is set at, say, 1.5, even if the sequence identity is low. The table below can be used as a guide as to the default RMS value corresponding to ID.
If you construct a model by homology modelling, remember that the RMS error you expect is essentially the error you expect from the template structure (if not worse!). So specify the sequence identity of the template, not of the homology model.
Only the model with the highest sequence identity is reported in the output pdb file. Also, HETATM cards in the input pdb file are ignored in the calculation of the structure factors for the ensemble, but are carried through to the output pdb file. Thus, the phases on the output mtz file (which come from the structure factors of the ensemble) do not correspond to those that would be calculated from the output pdb file, when there is more than one pdb file in an ensemble and/or the pdbfile(s) have HETATM records.
| #50 | #100 | #200 | #300 | #400 | #600 | #850 | #1000 | #1500 | #2000 | |
|---|---|---|---|---|---|---|---|---|---|---|
| ID=0% | 1.579 | 1.689 | 1.875 | 2.030 | 2.164 | 2.391 | 2.625 | 2.748 | 3.093 | 3.375 |
| ID=10% | 1.356 | 1.451 | 1.610 | 1.743 | 1.858 | 2.053 | 2.255 | 2.360 | 2.657 | 2.899 |
| ID=20% | 1.165 | 1.246 | 1.383 | 1.497 | 1.596 | 1.764 | 1.936 | 2.027 | 2.281 | 2.489 |
| ID=30% | 1.000 | 1.070 | 1.188 | 1.286 | 1.371 | 1.515 | 1.663 | 1.741 | 1.959 | 2.138 |
| ID=40% | 0.859 | 0.919 | 1.020 | 1.104 | 1.177 | 1.301 | 1.428 | 1.495 | 1.683 | 1.836 |
| ID=50% | 0.738 | 0.789 | 0.876 | 0.948 | 1.011 | 1.117 | 1.227 | 1.284 | 1.445 | 1.577 |
| ID=60% | 0.634 | 0.678 | 0.752 | 0.814 | 0.868 | 0.959 | 1.053 | 1.103 | 1.241 | 1.354 |
| ID=70% | 0.544 | 0.582 | 0.646 | 0.699 | 0.746 | 0.824 | 0.905 | 0.947 | 1.066 | 1.163 |
| ID=80% | 0.467 | 0.500 | 0.555 | 0.601 | 0.640 | 0.708 | 0.777 | 0.813 | 0.915 | 0.999 |
| ID=90% | 0.401 | 0.429 | 0.477 | 0.516 | 0.550 | 0.608 | 0.667 | 0.698 | 0.786 | 0.858 |
| ID=100% | 0.345 | 0.369 | 0.409 | 0.443 | 0.472 | 0.522 | 0.573 | 0.600 | 0.675 | 0.737 |
Coordinate Editing
HETATM/LIGANDS
Phaser ignores the scattering from HETATM records. The HETATM records are carried though to output with occupancy set to zero. Ligands will therefore not contribute to the scattering used for molecular replacement. The exceptions to this rule are the HETATM records for MSE (seleno-methionine) MSO (seleno-methionine selenoxide) CSE (seleno-cysteine) CSO (seleno-cysteine selenoxide) ALY (acetyllysine) MLY (n-dimethyl-lysine) and MLZ (n-methyl-lysine) which are used in the scattering and carried through to output with their original occupancy. If you wish to include any HETATM records in the scattering the record name use the keyword ENSE modlid HETATOM ON
WATER
Water molecules (identified by the residue name OW WAT HOH H2O OH2 MOH WTR or TIP) are deleted from the pdb file on input, are not used in the scattering and are not carried through to file output. If you want to retain water molecules you will need to change the residue name to something other than this (e.g. WWW) so that the atoms are not identified as water. To include the water molecules in the scattering, the HETATM records will also have to be changed to ATOM records as described above.
Building an Ensemble from Electron Density
When using density as a model, it is necessary to specify both the extent (x,y,z limits) of the cut-out region of density, and the centre of this region. With coordinates, Phaser can work this out by itself. This information is needed, for instance, to decide how large rotational steps can be in the rotation search and to carry out the molecular transform interpolation correctly. In the case of electron density, the RMS value does not have the same physical meaning that it has when the model is specified by atomic coordinates, but it is used to judge how the accuracy of the calculated structure factors drops off with resolution. A suitable value for RMS can be obtained, in the case of density from an experimentally-phased map, by choosing a value that makes the SigmaA curve fall off with resolution similarly to the mean figures-of-merit. In the case of density from an EM image reconstruction, the RMS value should make the SigmaA curve fall off similarly to a Fourier correlation curve used to judge the resolution of the EM image.
For detailed information, including a tutorial with example scripts, see Using density as a model
How to Define Composition
The composition defines the total amount of protein and nucleic acid that you have in the asymmetric unit. It is very important to include everything in the composition, not just the components that you are searching for, because Phaser needs to know what fraction of the total scattering is accounted for by each model. For the options that specify the size of a particular component (sequence, number of residues, molecular weight), you can separately define several components of the composition of the asymmetric unit and Phaser will just add them up. Note that, for these options, you can specify the composition of one copy of a component and also say how many copies of that component are expected to be present. You can also mix compositions entered by sequence, number of residues and molecular weight. When the composition is checked, Phaser will check for the plausibility of the composition you have specified, as well as multiples of that composition.
Default Composition
For convenience, the composition defaults to 50% protein scattering by volume (the average for protein crystals). It is better to enter it explicitly, even if only to check that you have correctly deduced the probable content of your crystal. If your crystal has higher or lower solvent content than this, or contains nucleic acid, then the composition should be entered explicitly.
Composition by Sequence
The composition is calculated from the amino acid sequence of the protein and the base sequence of the nucleic acid in fasta format.
Composition by Atom
Individual atoms can be added to the composition. This allows the explicit addition of heavy atoms in the structure, e.g. Fe atoms.
Composition by Solvent Content
Scattering is determined from the solvent content of the crystal, assuming that the crystal contains protein only, and the average distribution of amino acids in protein. If your crystal contains nucleic acid or your protein has an unusual amino acid distribution then the composition should be entered explicitly using the MW or sequence options.
Composition by Number of Residues in ASU
Scattering is determined from the number of residues in the asymmetric unit, assuming that the crystal contains protein only or nucleic acid only, and assuming an average distribution of residues for either. If your crystal contains a mixture then the composition should be entered explicitly using the MW or sequence options. If your crystal has an unusual residue distribution then the composition should be entered explicitly using the sequence options.
Composition by Molecular Weight
The composition is calculated from the molecular weight of the protein and nucleic acid assuming the protein and nucleic acid have the average distribution of amino acids and bases. If your protein or nucleic acid has an unusual amino acid or base distribution the composition should be entered by sequence. You can mix compositions entered by molecular weight with those entered by sequence.
Composition by Percentage Scattering
The fraction scattering of each ensemble can be entered directly. The fraction scattering of each ensemble is normally automatically worked out from the average scattering from each ensemble (calculated from the pdb files if entered as coordinates, or from the protein and nucleic acid molecular weights if entered as a map) divided by the total scattering given by the composition, but entering the fraction scattering directly overrides this calculation. This option is for use when the pdb files of the models in the ensemble are unusual e.g. consist only of C-alpha atoms, or only of hydrogen atoms (as in the CLOUDS method for NMR).
How to Define Searches
Phaser does not compare sequences you specify in the composition with the models you specify as ensembles, so you have to specify separately the number of copies of a particular sequence that you expect to be found in the asymmetric unit of your crystal and the number of copies of each ensemble you want to place in the asymmetric unit. By default, Phaser will search first for ensembles expected to yield the highest signal in the MR search (as judged by the expected LLG or eLLG calculation); if that fails to result in a clear solution, different search orders will be tested automatically. For that reason, it does not normally matter which order you use to specify the searches. There is an option to override Phaser's automatic choice of search order, but this will only rarely be useful. It is best to specify the searches for everything that you hope to find in the MR calculation in one job, as that gives Phaser the greatest scope to optimise the calculation. Note that if your crystal possesses translational non-crystallographic symmetry (tNCS), you should be searching for a number of copies of each ensemble divisible by the order of the tNCS (i.e. the number of molecules that should be related by repeated application of a translation vector).
How to Define Solutions
Phaser writes out files ending in ".sol" and ".rlist" that contain the solution information from the job. The root of the files is given by the ROOT keyword. By default, the root filename is PHASER. These files can be read back into subsequent runs of Phaser to build up solutions containing more than one molecule in the asymmetric unit.
"PHASER.sol" files are generated by all modes (rotation function modes with VERBOSE output), and contain the current idea of potential molecular replacement solutions.
"PHASER.rlist" files are generated by the rotation function modes, and are used as input for performing translation functions.
For simple MR cases you don't really need to know how to define molecular replacement solutions. However, for difficult cases you might need to edit the files "PHASER.sol" and "PHASER.rlist" files manually
"sol" Files
SOLUtion 6DIM keywords describe Ensembles that have been oriented by a rotation search and positioned by a translation search. Each Ensemble in the asymmetric unit has its own SOLUtion keyword. When more than one (potential) molecular replacement solution is present, the solutions are separated with the SOLUTION SET keywords.
"rlist" Files
These files define a rotation function list. The peak list is given with a series of SOLUtion TRIAl keywords.
If a partial solution is already known, then the information for the currently "known" parts of the asymmetric unit is given in the form used for the PHASER.sol file, followed by the list of trial orientations for which a translation function is to be performed.
Fixed partial structure
If you have the coordinates of a partial solution with the pdb coordinates of the known structure in the correct orientation and position, then you can force Phaser to use these coordinates. Use the SOLUTION keyword to fix a rotation of 0 0 0 and a position of 0 0 0 for these coordinates.
How to Select Peaks
The selection of peaks saved for output in the rotation and translation functions can be done in four different ways.
- Select by Percentage
- Percentage of the top peak, where the value of the top peak is defined as 100% and the value of the mean is defined as 0%.
- Default, cutoff=75%. This criteria has the advantange that at least one peak (the top peak) always survives the selection. If the top solution is clear, then only the one solution will be output, but if the distribution of peaks is rather flat, then many peaks will be output for testing in the next part of the MR procedure (e.g. many peaks selected from the rotation function for testing with a translation function).
- Select by Z-score
- Number of standard deviations (sigmas) over the mean (the Z-score).
- Absolute significance test. Not all searches will produce output if the cutoff value is too high (e.g. 5 sigma).
- Select by Number
- Number of top peaks to select.
- If the distribution is very flat then it might be better to select a fixed large number (e.g. 1000) of top rotation peaks for testing in the translation function.
- No selection
- All peaks are selected.
- Enables full 6 dimensional searches, where all the solutions from the rotation function are output for testing in the translation function. This should never be necessary; it would be much faster and probably just as likely to work if the top 1000 peaks were used in this way.
Peaks can also be clustered or not clustered prior to selection in steps 1 and 2.
- Clustering Off
- All high peaks on the search grid are selected
- Clustering On
- Points on the search grid with higher neighbouring points are removed from the selection
How to Control Output
The output of Phaser can be controlled with optional keywords.
The ROOT keyword is not compulsory (the default root filename is "PHASER"), but should always be given, so that your jobs have separate and meaningful output filenames.
The TOPFiles keyword controls the number of potential MR solutions for which PDB and (in the appropriate modes) MTZ files are produced.
For the MR_AUTO, MR_RNP and MR_LLG modes, unless HKLOut OFF is given as an optional keyword, Phaser produces an MTZ file with "SigmaA" type weighted Fourier map coefficients for producing electron density maps for rebuilding.
| MTZ Column Labels | Description |
|---|---|
| FWT/PHWT | Amplitude and phase for 2m|Fobs|-D|Fcalc| exp(iαcalc) map |
| DELFWT/PHDELWT | Amplitude and phase for m|Fobs|-D|Fcalc| exp(iαcalc) map |
| FOM | m, analogous to the "Sim" weight, to estimate the reliability of αcalc |
| HLA/HLB/HLC/HLD | Hendrickson-Lattman coefficients encoding the phase probability distribution |
Translational Non-crystallographic Symmetry
*Warning* Solution by MR in the presence of translational non-crystallographic symmetry is not fully automated.
Phaser calculates correction factors for the expected intensities in the presence of translational non-crystallographic symmetry (tNCS), and is able to solve structures with complex patterns of tNCS. However, the use of Phaser in the presence of tNCS requires the nature of the tNCS to be understood by the user. In simple cases, solution is no more difficult than solution without tNCS, but in complex cases, separate Phaser runs with tNCS turned on and off, and/or the use of different tNCS vectors, may be necessary.
The output of Phaser will help the user in detecting and understanding the tNCS, but the tNCS is not completely characterised by Phaser. The default behaviour may or may not be correct for the particular crystal under study.
Characterization of the tNCS involves understanding the number of copies of the molecule in the asymmetric unit and the translation vectors between them. Molecules related by a tNCS vector will have an associated peak in the native Patterson. Phaser calculates the native Patterson (MODE TNCS) and lists the peaks that are more than 20% of the origin peak. Any given crystal with tNCS may have one or more peaks meeting this criteria.
Default tNCS detection and correction
Documentation for Phaser-2.7.16 and above
No tNCS
No tNCS correction is applied by default if there is
- no peak in the native Patterson
- more than one peak in the native Patterson over 20% of the origin and these peaks are not all the result of a commensurate modulation
Pairs of molecules
By default, if Phaser detects a peak in the native Patterson then Phaser will search for molecules in pairs related by the tNCS vector given by the peak in the native Patterson.
This will be the correct behaviour if and only if there are an even number of copies of the molecule in the asymmetric unit, clustered into two groups related by a single tNCS vector. There will only be one significant peak in the native Patterson. Fortunately, this is a reasonably common scenario.
Phaser refines the relative orientation of the molecules in the two groups (rotations of up to 10 degrees will still give rise to a significant native Patterson peak) and uses this information to generate expected intensity factors for the reflections. Solution should be straightforward, with the usual caveat for MR that there is a sufficiently good model.
Where there is a single peak in the native Patterson, it is often located at a position half way along a unit cell axis or diagonal, representing a pseudo-halving of the unit cell dimensions. However, Phaser is by no means restricted to these sorts of pseudo-cells in its handling of two-fold tNCS, and the tNCS vector can be in a general position.
Non-default tNCS correction
tNCS involving multimers
It is common for tNCS to arise through the combination of a crystallographic symmetry element and a nearly-parallel internal symmetry of a multimer. For example, if the local 2-fold axis of a dimer is parallel to a crystallographic 2-fold, the symmetry-related copy of monomer "B" will end up related by a pure translation to the original copy of monomer "A" from the dimer. Because Phaser handles tNCS by placing multiple copies related by the tNCS translation, the best approach in this example would be to search for two copies of the monomer. In most cases, the alternative approach of telling Phaser to ignore the tNCS will not work as well, but it may be worth testing this approach.
Higher order tNCS
Frequently, tNCS does not associate 2 clusters of molecules in the asymmetric unit, but rather there are 3 or more (n) clusters of molecules associated by a series of vectors that are multiples of 1, 2, 3 ... (n-1) times a basic translation vector. Where n times the basic translation vector equates to (very close to) integer multiples of unit cell axes, the tNCS represents a pseudo-cell, and this case is known as commensurate modulation.
Phaser attempts to automatically detect commensurate modulation. The peaks of the native Patterson are analyzed to find the n-fold relationship. The series will not generally have all peaks the same height. Lower peaks in the series represent relationships where the relative rotations between related molecules are larger. Missing peaks in the series may be below the default 20% of origin cut-off. This can be lowered with TNCS PATT PERCENT <x>
Phaser then sets TNCS NMOL <n> and the vector for the tNCS, and searches for ensembles in multiples of NMOL.
When there are more than two molecules related by tNCS, Phaser does not refine the orientations between the molecules related by the tNCS.
However, as for two-fold tNCS, Phaser is not restricted to these sorts of pseudo-cells and the basic tNCS vector can be in a general position, as can the number of copies.
The automatic detection may not give the true tNCS relationship. For example, the true commensurate modulation may be a factor of the NMOL automatically detected by Phaser, or there may not be commensurate modulation at all, or commensurate modulation may not be found with the default Pattesron peak height cutoff. In difficult cases, please inspect the Patterson for peaks.
Complex tNCS
If there are many molecules in the asymmetric unit but they are not all related by tNCS, or there are sub-groups of molecules related by different tNCS vectors, then the modulations of the expected intensities due to the tNCS will be much less significant than the cases described above. In these cases it is possible that structure solution will be achieved without any tNCS correction factors being applied. Indeed, searching for all the copies as tNCS-related multiples when some molecules are not related by tNCS will cause structure solution to fail. To turn off the automatic detection and use of tNCS use the keyword TNCS USE OFF.
If turning off the TNCS correction factors fails to give a solution, then a good approach is to proceed step-wise. Consider the highest native Patterson peak first and determine that nature of the tNCS associated with it. Use the appropriate correction factors to locate all the molecules with this tNCS. Then take the second independent native Patterson peak and apply the correction factors associated with it to find the second set of molecules, fixing the first, etc. Finally, turn TNCS off to find any orphan molecules.